LINES OF INVESTIGATION
Differential Expression during Lumen Formation in MDCK
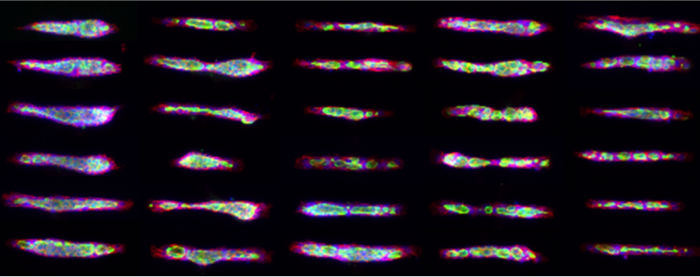
The general aim of our research is to characterize new proteins required for epithelial lumen formation, and to further understand their function and molecular mechanism, using a combination of 3D organotipic epithelial cultures, and an in vivo model of epithelia morphogenesis. Is lumen formation regulated at expression levels? Studies performed mainly in Drosophila melanogaster epithelia have shown that transcriptional promotion and repression control the expression of multiple genes involved in apical polarity and luminal development, including membrane trafficking pathways, polarity complex, etc [6, 7]. The general aim of our research is characterize new proteins required for the formation of the lumen in epithelial morphogenesis, and further understand their function and molecular mechanism. Traditionally, the analysis of epithelial polarity and morphogenesis has been performed using either in vitro culture cell lines in monolayers (2D), or in vivo models. However, we are using a novel approach consisting in a functional screening using a combination of 3D-organotypic epithelial cultures, and an in vivo model for epithelia morphogenesis. In particular, we are using the 3D-MDCK system for the systematic analysis and the functional characterization of the proteins upregulated in lumen formation. In this way, we are obtaining crucial information about the temporal and spatial regulation of the basic machinery and molecular mechanisms that regulate epithelial polarity. For instance, timely regulation of Synaptotagmin-like protein expression modulates the activity of a t-SNARE, syntaxin-3, which constitutes the essential subunit of the fusion machinery at the apical plasma membrane [8].
Novel Mechanisms and Signaling Pathways Involved in Tube Morphogenesis
Our initial functional screening of lumen-formation proteins focused on a limited number of differentially expressed proteins, which were flagged by bioinformatic methods for containing putative polarity-regulatory functions. These were selected either because they contained some domain with putative functions involved in signaling, cytoskeletal and lipid regulation, or because they were shown to interact (physically, genetically or bioinformatically) with proteins with known polarity functions. Appart from functionally describing these novel regulators, we’re currently carrying out additional wider screens to focus on other genetic elements which are differentially expressed during lumen formations. As readouts we are now using a subset of these induced proteins as a “polarity signature” for lumen forming cells.
Vesicle Trafficking and Lumen Formation in Zebrafish Gut Morphogenesis
The use of in vitro 3D organotypic systems, which serve to model epithelial organ morphogenesis in vivo, are generating exciting new insights on the coordinated development of apical–basal polarity in epithelia. However, they cannot reconstitute the complexity of the in vivo architecture, which includes different cell types, dynamic remodeling, and tissue homeostasis. For this reason, the use of in vivo systems would serve to validate and further characterize the phenotypes observed in vitro. The zebrafish epithelial morphogenesis system represents an excellent model to characterize (in vivo) the mechanisms for lumen formation identified in the 3D MDCK system [9]. To complement the in vitro approach with 3D-MDCK cysts, we are using the zebrafish epithelial morphogenesis system to address the role of proteins involved in lumen formation in vivo, and to further characterize the phenotypes observed in vitro. There are three epithelial tubes in zebrafish susceptible for this analysis: the gut, the neural tube and the pronephrotic ducts. For our experiments, we are using the gut and the pronephrotic ducts to conduct this analysis.
Adhesion-mediated regulation of epithelial morphogenesis and lumen formation
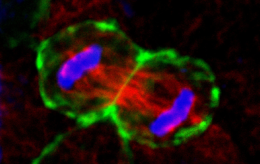
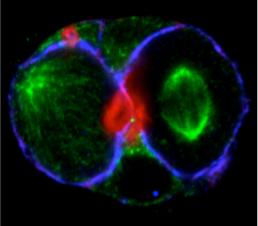
Micropatterns enable the functional analysis of adhesion in processes ranging from cell polarity and migration, to cell division and differentiation. Recent use of micropatterns has elucidated a role for cell confinement in ciliogenesis and stemness maintenance. In collaboration with French micropatterning technology pioneers, CYTOO, we have optimized micropatterning techniques for the culture of MDCK organoids, which serve to model epithelial organ morphogenesis in a more standardized fashion, enabling its use for the Pharma industry. Additionally, we are using micropatterns to evaluate the role of cell adhesion on lumen morphogenesis and epithelial signaling and differentiation. Our first data on this front has elucidated that laminin constitutes a major inhibitor of cell spreading and actin contractility, which modulates the organization of microtubules and centrosomes and correlates with apical membrane organization after cell division [10].
In summary, we are using cutting-edge methodologies such as multiphoton microscopy and organotypic 3-dimensional micropatterned cell cultures, combined with genomic and bioinformatic tools, to unravel the process of epithelial lumen formation and the acquisition of cell polarity. We expect to obtain key information on the machinery and molecular mechanisms that regulate these processes. Indeed, this subject is central to human health. Many human diseases, such as cancer, initiate or proceed with defects in epithelial organization machineries. A molecular understanding of tube morphogenesis will lead to new ways of preventing and treating these conditions, and is therefore, a major challenge for the future.
References
-
1. Bryant, D.M., and Mostov, K.E. (2008). From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9,
887-901.
-
2. Mostov, K., Su, T., and ter Beest, M. (2003). Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell
Biol 5, 287-293.
-
3. Lubarsky, B., and Krasnow, M.A. (2003). Tube morphogenesis: making and shaping biological tubes. Cell 112, 19-28.
-
4. Debnath, J., and Brugge, J.S. (2005). Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev
Cancer 5, 675-688.
-
5. O'Brien, L.E., Zegers, M.M., and Mostov, K.E. (2002). Opinion: Building epithelial architecture: insights from three-
dimensional culture models. Nat Rev Mol Cell Biol 3, 531-537.
-
6. Kerman, B.E., Cheshire, A.M., Myat, M.M., and Andrew, D.J. (2008). Ribbon modulates apical membrane during tube
elongation through Crumbs and Moesin. Dev Biol 320, 278-288.
7. Martin-Belmonte, F., Gassama, A., Datta, A., Yu, W., Rescher, U., Gerke, V., and Mostov, K. (2007). PTEN-mediated
apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383-397.
-
8. Galvez-Santisteban, M., Rodriguez-Fraticelli, AE, et al. (2012). Synaptotagmin-like proteins control the formation of a single apical membrane
domain in epithelial cells. Nat Cell Biol 22, 838-49.
-
9. Bagnat, M., et al. (2007). Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol 9, 954-60.
-
10. Rodriguez-Fraticelli, AE, et al. (2012). Cell confinement controls lumen formation and centrosome positioning during epithelial morphogenesis.
J Cell Biol.